Grainger Laboratory
 Wnt Signaling in Hematopoietic Stem Cell Development and Cancer
Wnt Signaling in Hematopoietic Stem Cell Development and Cancer
If the human body is an ongoing construction project, then stem cells are its all-purpose building materials. These biological “blank slates” give rise to all the specialized cell types needed to assemble and power the human body, from skin cells to heart cells and everything in between.
This incredible ability to differentiate makes stem cells central players in normal development as well as in diseases like cancer. Understanding exactly how stem cells work — and what happens when the processes that govern stem cell development go awry — holds great promise for better understanding health and developing new treatments for cancer and other diseases. To this end, the Grainger Lab investigates the mechanisms that govern how stem cells are made, how they are maintained and — when things go wrong — how they can become cancerous.
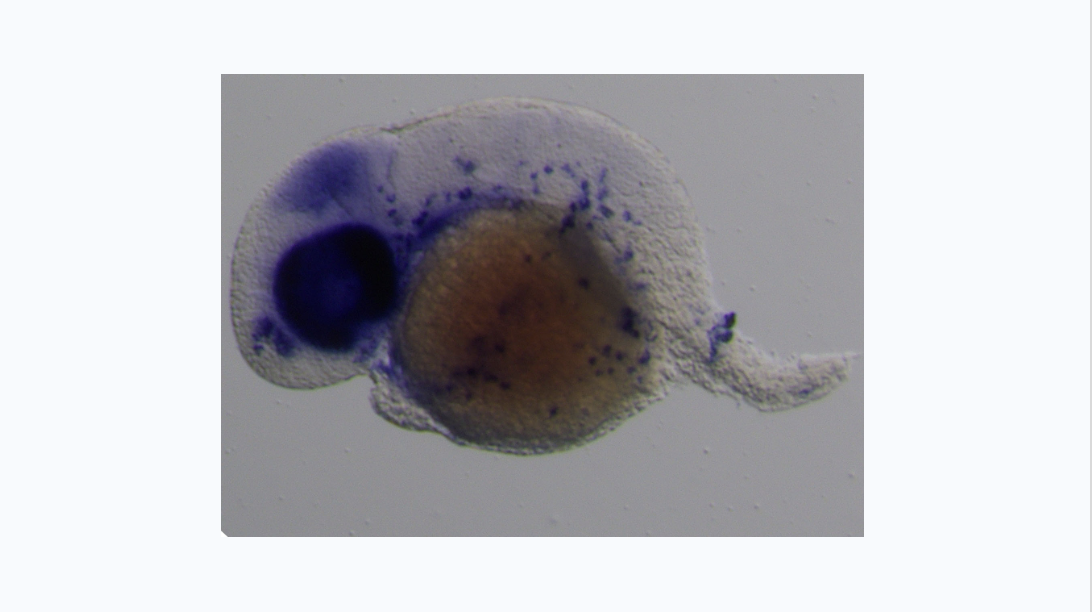
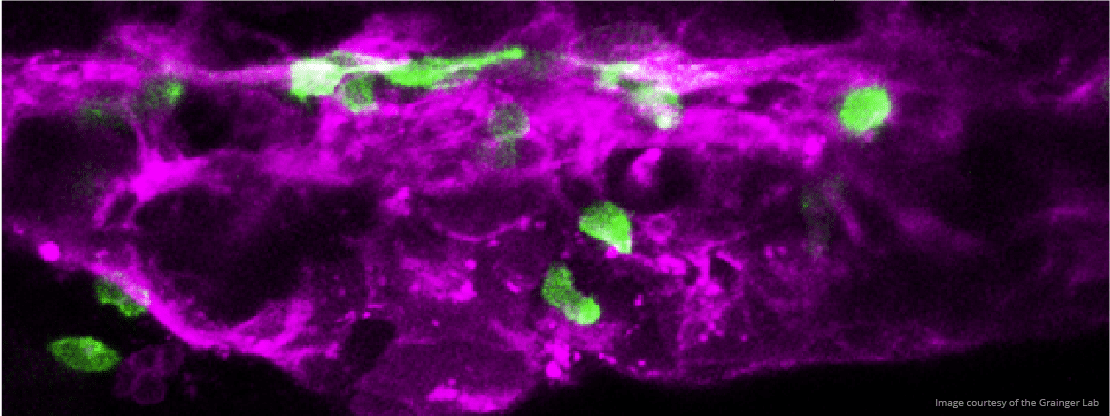
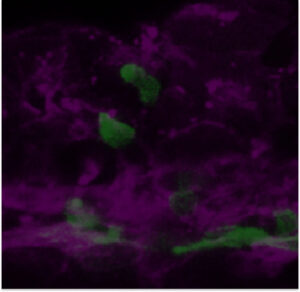
We are particularly interested in the Wnt pathway, a cellular communication channel that is central to a plethora of processes during embryonic development, tissue regeneration and carcinogenesis. In a previous study by our lab (Grainger and Richter, 2016, Cell Reports), we determined that a very specific Wnt signal (Wnt9a) is required for the amplification of hematopoietic stem cells earlier in development than was previously thought. In our follow up study (Grainger, 2019, Nature Cell Biology), we found that the specific Wnt9a signal is received by Fzd9b and that signaling specificity is conferred by the epidermal growth factor receptor (EGFR). We are now working to learn more about this signaling axis.
We’re also interested in how non-canonical Wnt signals arise. Using zebrafish and cell culture as models, we’re investigating if there are specific functions for Wnt ligands or Fzd receptors in stem cell biology.
Our Impact
We’re raising thousands to save millions.
We’re turning hope into action for the millions of people around the world affected by diseases like cancer and Parkinson’s. Find out how you can help us make a difference.
- 122 peer-reviewed papers published in 2024, 63 of which were in high-impact journals
- 15 VAI-SU2C Epigenetics Dream Team clinical trials launched to date
- 10 clinical trials co-funded by VAI & Cure Parkinson's (out of 41 total International Linked Clinical Trials Program trials)
Stephanie Grainger, Ph.D.
Assistant Professor, Department of Cell Biology
Areas of Expertise
Wnt signaling, stem cell development, cancer, hematopoiesis
Biography
Dr. Stephanie Grainger is an authority on the biological underpinnings of how stem cells develop, and how these processes can go awry during cancer. She earned her Ph.D. in Cellular and Molecular Medicine from the University of Ottawa, where she investigated the role of Cdx transcription factors in intestinal development. She then completed a postdoctoral fellowship in the labs of Dr. David Traver and Dr. Karl Willert at University of California, San Diego. Her research during this time sought to understand the role of Wnt signaling in hematopoietic stem cell development — work that she continued as an assistant professor at San Diego State University. During this time, she discovered a novel stage of hematopoietic stem cell development, which is driven by a specific Wnt cue. Furthermore, she discovered a novel mechanism of Wnt signal regulation. In 2021, Dr. Grainger joined Van Andel Institute’s Department of Cell Biology.
Professional Memberships
- Society for Developmental Biology
- American Heart Association
- International Society for Experimental Hematology
- American Society for Hematology
- American Society for Cell Biology
Selected Publications
View Dr. Grainger’s NCBI bibliography here.
* denotes equal contribution
# denotes co-corresponding authors
Ensing J, Ide AD, Gilliland C, Tsurho V, Rudisel E, Caza I, Stratman AN, Lanning NJ, Grainger S. 2025. The E3 ubiquitin Trip12 semi-selectively attenuates Wnt signaling. iScience 28(10).
Tsurho V, Gillilan C, Ensing J, VanSickle EA, Lanning NJ, Mark PR, Grainger S. 2025. A zebrafish model of nicotinamide adenine dinucleotide (NAD+) deficiency-derived congenital disorders. Dev Biol 271–277.
Ide AD, Carpenter KA, Elaswad MT, Opria K, Marcellin K, Gilliland C, Grainger S. 2025. Secreted frizzled-related protein 1a regulates hematopoietic development in a dose-dependent manner. Dev Biol 1–12.
Ide AD, Grainger S. 2025. WNT9A and WNT9B in development and disease. Differentiation.
Abello J, Yin Y, Zhao Y, Maurer J, Lee J, Bodell C, Richee J, Clevenger AJ, Burton Z, Goeckel ME, Lin M, Grainger S, Halabi CM, Raghavan SA, Sah R, Stratman AN. 2025. Endothelial cell Piezo 1 promotes vascular smooth muscle cell differentiation on large arteries. Eur J Cell Biol 104(1).
Nguyen N, Carpenter KA, Ensing J, Gilliland C, Rudisel EJ, Mu EM, Thurlow KE, Triche Jr. TJ, Grainger S. 2024. EGFR-dependent endocytosis of Wnt9a and Fzd9b promotes β-catenin signaling during hematopoietic stem cell development in zebrafish. Sci Sig 17(832).
Molina B*, Chavez J*, Grainger S. 2020. Zebrafish models of acute leukemias: Current models and future directions. Wiley Interdiscip Rev Dev Biol:e400.
Gumber D*, Do M*, Suresh Kumar N, Wu CCN, Cruz LS, Grainger S, Carson D, Gaasterland T, Willert K, 2020. Selective activation of FZD7 promotes mesendodermal differentiation of human pluripotent stem cells. eLife 9:e63060.
Espanola SG, Song H, Ryu E, Saxena A, Kim ES, Manegold JE, Nasamran CA, Sahoo D, Oh CK, Bickers C, Shin U, Grainger S, Park YH, Pandolfo L, Kang MS, Kang S, Myung K, Cooper K, Yelon D, Lee Y, Traver D. 2020. Hematopoietic stem cell-dependent Notch transcription is mediated by P53 through the histone chaperone Supt16h. Nat Cell Bio 22:1411–1422.
Grainger S, Nguyen N, Richter J, Setayesh J, Lonquich B, Oon CH, Wozniak JM, Barahona R, Kamei C, Houston J, Carrillo-Terrazas M, Drummond I, Gonzalez D, Willert K, Traver D. 2019. EGFR confers exquisite specificity of Wnt9a-Fzd9b signaling in hematopoietic stem cell development. Nat Cell Bio 21(6):721–730.
Bickers C, Espanola S, Grainger S, Pouget C, Traver D. 2018. Comparison of snai2 morpholino and mutant phenotypes. PLoS One 13(9):e0202747.
Markmiller RL*, Schaffer AE*, Eggans VR, Zaki MS, Grainger S, Sathe S, Van Nostrand EL, Schlachetzki Z, Rosti B, Akizu N, Scott E, Heckman LD, Rosti RO, Dikoglu E, Gregor A, Guemez-Gamboa A, Musaec D, Mande R, Widjaja W, Shaw TL, Markmiller S, Marin-Valencia I, Foulds N, Dobyns WB, Chi N, Traver D, Spaccini L, Bova S, Gabriel SB, Gunel M, Valente EM, Bennett EJ, Yeo GW, Baas F, Lykke-Andersen J#, Gleeson JG#. 2017. Biallelic mutations in the 3’ exonuclease TOE1 cause Pontocerebellar Hypoplasia Type 7 and result in snRNA processing defects. Nat Genet 49(3):457–464.
Grainger S, Lonquich B, Oon CH, Nguyen N, Willert K#, Traver D#. 2017. CRISPR guide RNA validation in vitro. Zebrafish 14(4):383–386.
Grainger S*, Richter J*, Espin-Palazon R, Pouget C, Lonquich B, Wirth S, Grassme KS, Herzog W, Swift MR, Weinstein BM, Willert K#, Traver D#. 2016. Wnt9a is required for the aortic amplification of nascent hematopoietic stem cells. Cell Rep 17(6) 1595 –1606.
Hryniuk A*, Grainger S*, Savory JGA, Lohnes D, 2014. Cdx1 and Cdx2 function as tumor suppressors. J. Biol Chem 289(48):33343–33354.
Grainger S, Hryniuk A, Lohnes D. 2012. Cdx1 and Cdx2 exhibit transcriptional specificity in the intestine. PLoS One 8(1):e54757.
Lafontaine C*, Grainger S*, Hess BL, Béland M, Lohnes D. 2012. Cdx1 interacts physically with a subset of Hox proteins. Biochemistry J 51(48):9698–9705.
Hryniuk A, Grainger S , Savory JGA, Lohnes D. 2012. Cdx is required for maintenance of intestinal identity in the adult. Dev Biol 363:426–437.
Grainger S*, Lam J*, Savory JGA, Mears AJ, Rijli F, Lohnes D. 2011. Cdx regulates Dll1 in multiple lineages. Dev Biol 361, 1–11.
Grainger S, Savory JGA, Lohnes D. 2010. Cdx2 regulates patterning of the intestinal epithelium. Dev Biol 339:155–165.
Savory JGA, Pilon N, Grainger S, Sylvestre JR, Beland M, Houle M, Oh K, Lohnes D. 2009. Cdx1 and Cdx2 are functionally equivalent in vertebral patterning. Dev Biol 330:114–122.


Charles J. Bradfield
Aquatics Technician, Grainger Laboratory


Jessica Ensing, B.S.
Assistant Research Technician, Department of Cell Biology

Stephanie Grainger, Ph.D.
Assistant Professor, Department of Cell Biology
Wnt Signaling in Hematopoietic Stem Cell Development and Cancer

Carla Gilliland, B.S.
Research Technician, Department of Cell Biology


Michelle Minard, B.S.
Senior Administrative Assistant II

Nate Lanning
Senior Research Scientist, Department of Cell Biology

Mae Rydingsward
Laboratory Aide, Department of Cell Biology

Elizabeth VanSickle, M.S.
Guest Ph.D. Student
GRAINGER LABORATORY MASCOT
Nugget, Ph.D.
BIOGRAPHY
Education: Ph.D. in Fishery Science, Van Andel Institute Graduate School
Favorite Signaling Molecule: Wnt3a
Favorite Pastimes: Sunbathing at the beach